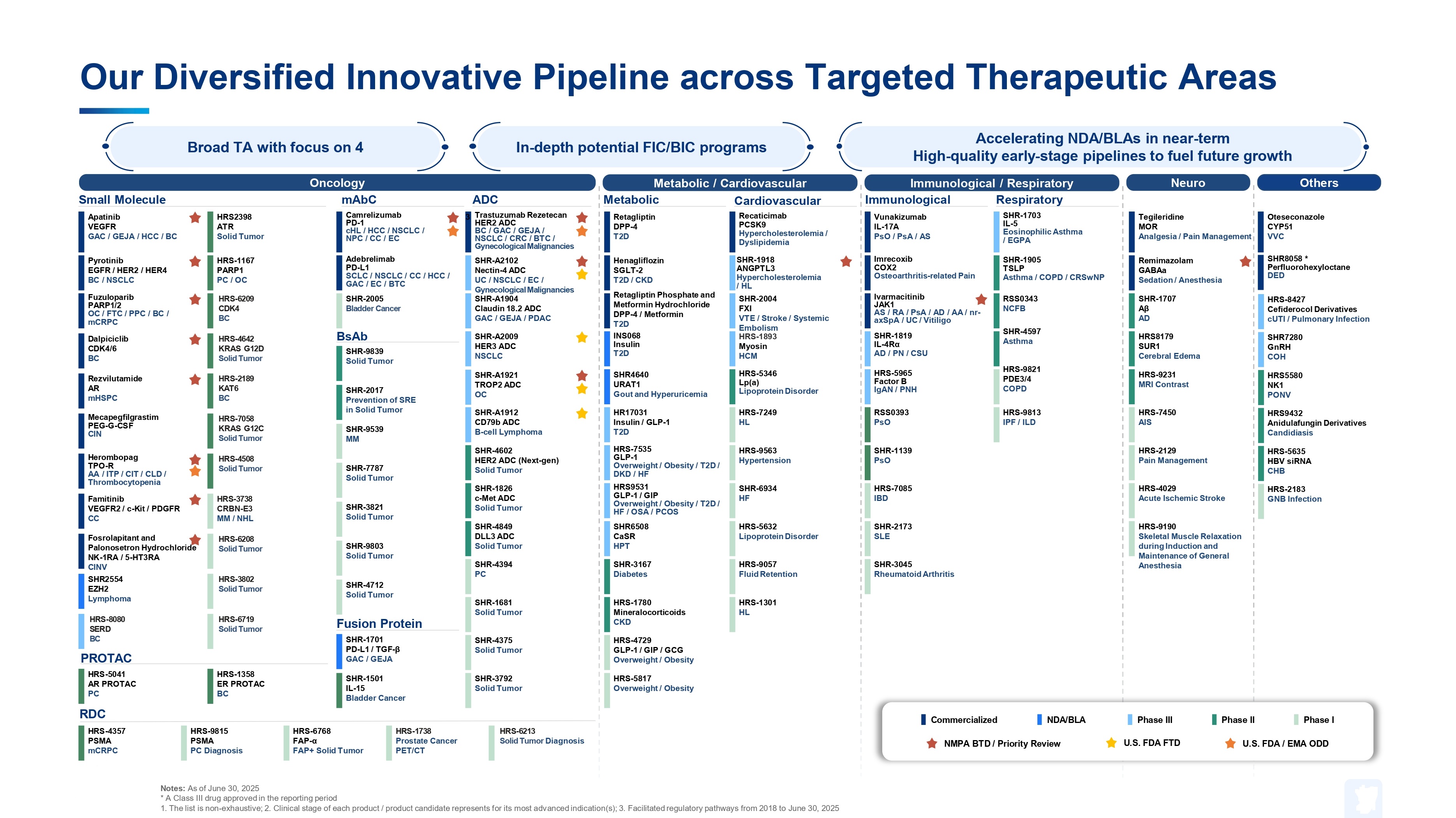
Pipeline
R&D PIPELINE
We established a diversified and comprehensive R&D pipeline covering oncology, autoimmune diseases, pain management, cardiovascular diseases, metabolic diseases, infectious diseases, respiratory system diseases, hematological diseases, neurological diseases, ophthalmology, etc.
Major R&D Pipelines
-
24
New Molecular Entity drugs marketed in China
-
5
Other innovative drugs marketed in China
-
100
+
In-House Innovative Pharmaceuticals under Clinical Development
-
400
+
Ongoing Clinical Studies
-
20
+
Ongoing International Clinical Studies
*By Jun, 2025